Research Site
Discover the Power of Knowledge at Vitality Cove’s Dedicated Research Site – Because Informed Wellness Leads to Better Health Outcomes
Treatment of skin aging with topical estrogens
https://pubmed.ncbi.nlm.nih.gov/8876303/
J B Schmidt 1, M Binder, G Demschik, C Bieglmayer, A Reiner
Affiliations Expand
PMID: 8876303 DOI: 10.1111/j.1365-4362.1996.tb03701.x
The study titled "Treatment of skin aging with topical estrogens" by Schmidt et al. (1996) investigated the effects of topical estrogen application on skin aging. The researchers found that topical estrogens improved several parameters of aged skin including increased skin thickness, enhanced collagen content, and improved skin moisture. These results suggest that estrogen therapy can positively influence skin structure and function, potentially reversing or mitigating signs of skin aging. The study supports the role of estrogens in maintaining skin health, particularly in postmenopausal women experiencing estrogen deficiency
The Gut-Brain Axis
1. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Brain Function
Conclusion: Emerging evidence indicates that gut microbiota is important in the regulation of brain activity and cognitive functions. Microbes mediate communication among the metabolic, peripheral immune, and central nervous systems via the microbiota-gut-brain axis. However, it is not well understood how the gut microbiome and neurons in the brain mutually interact or how these interactions affect normal brain functioning and cognition.
https://pubmed.ncbi.nlm.nih.gov/34205336/
2. Neurotransmitter Modulation by the Gut Microbiota in Anxiety and Depression
Conclusion: The gut microbiota has a clear impact on neurotransmitters including serotonin, dopamine, GABA, and glutamate. Mental disorders such as schizophrenia, depression, anxiety disorders, and autism spectrum disorder are associated with gut microbiota. Therapies influencing the gut microbiota, including probiotics, prebiotics, and fecal microbiota transplant, could help in the treatment of mental illnesses.
3. The Role of Gut Microbiota in Anxiety, Depression, and Other Mental Disorders
Conclusion: Several gut microbiota, especially Firmicutes and Bacteroidetes, are demonstrated to affect mental health through the microbiota-gut-brain axis. The number of individuals experiencing mental disorders (e.g., anxiety and depression) has significantly risen in recent years. Therefore, it is essential to seek prevention and treatment strategies for mental disorders.
https://pubmed.ncbi.nlm.nih.gov/37513676/
4. Mental Disorders Linked to Crosstalk between The Gut Microbiome and the Brain
Conclusion: Compositional changes in the gut microbiome are closely associated with several mental disorders, which can be improved by controlling the gut microbiome. However, some issues have yet to be conclusively addressed, especially the causality between the gut microbiome and mental disorders.
https://pubmed.ncbi.nlm.nih.gov/33139585/
5. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health
Conclusion: Clinical, epidemiological, and immunological evidence suggest that enteric microbiota extensively and profoundly influences the gut-brain relationship (i.e., mental state, emotional regulation, neuromuscular function, and regulation of the HPA).
6. From Gut Dysbiosis to Altered Brain Function and Mental Illness
Conclusion: Although an association between enteropathy and certain psychiatric conditions has long been recognized, it now appears that gut microbes represent direct mediators of psychopathology. Here, we examine roles of gut microbiome in shaping brain development and neurological function, and the mechanisms by which it can contribute to mental illness.
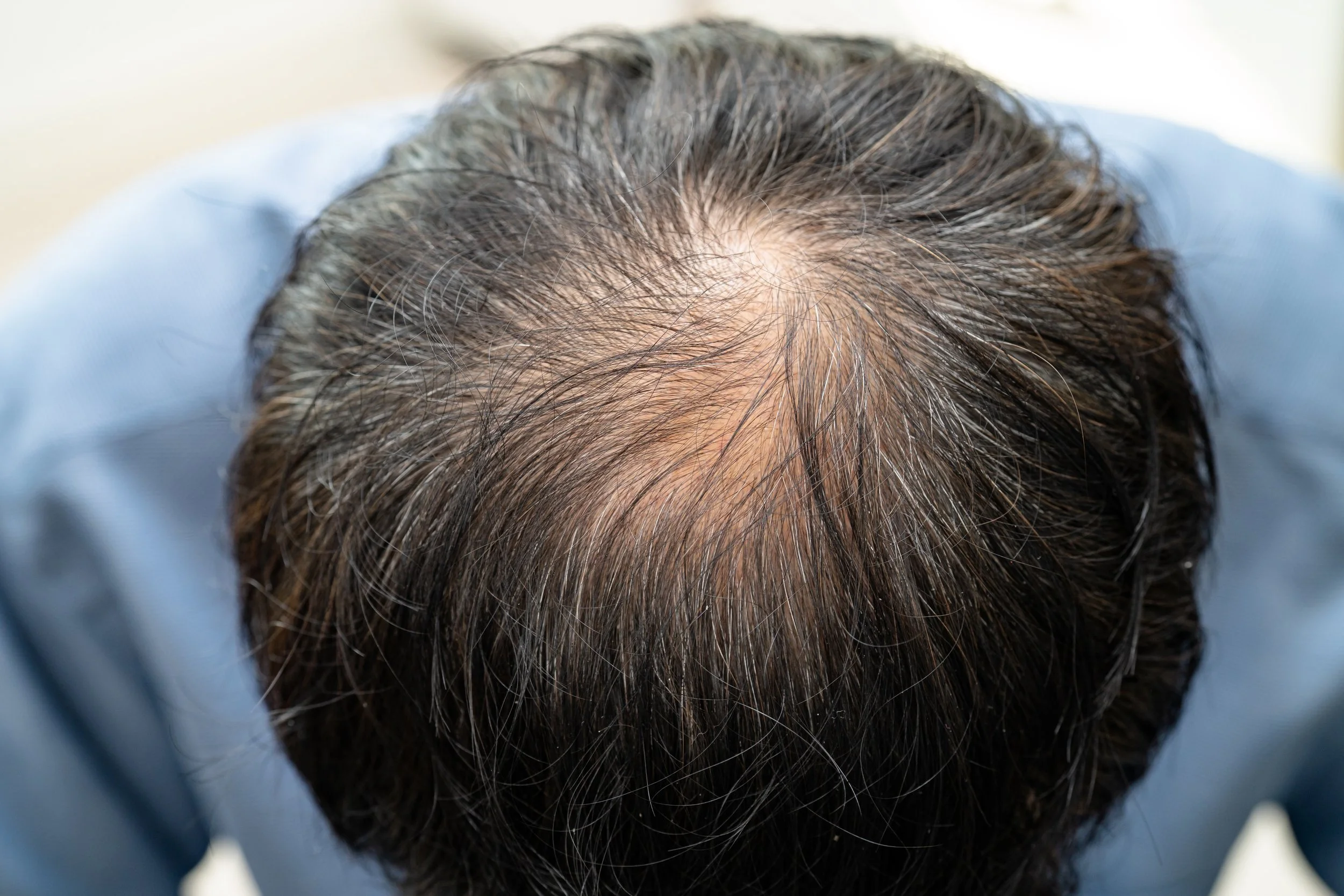
The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial
In the randomized placebo-controlled trial conducted by Gentile et al., titled The Effect of Platelet-Rich Plasma in Hair Regrowth, the authors demonstrated that platelet-rich plasma (PRP) treatment significantly improved hair density and thickness compared to placebo in patients with androgenetic alopecia. The study highlights PRP as a promising therapeutic option for hair regrowth by promoting follicular proliferation and enhancing the dermal papilla cell activity. (Gentile et al., 2025)
Study Design: Randomized, placebo-controlled trial evaluating the effect of platelet-rich plasma (PRP) on hair regrowth.
Participants: Individuals experiencing hair thinning or early stages of hair loss.
Intervention: PRP injections administered at specified intervals compared to placebo injections with a neutral solution.
Duration: Follow-up period ranging from several weeks to months to assess hair regrowth outcomes.
Primary Measures:
Hair count increase
Hair density improvements
Patient satisfaction and visual assessment by clinicians
Findings:
Significant increase in hair density and count observed in the PRP group compared to placebo.
Noticeable improvement in hair thickness and scalp coverage reported by participants receiving PRP.
Minimal adverse effects; procedure generally well-tolerated.
Conclusion:
Platelet-rich plasma therapy demonstrates a positive effect on promoting hair regrowth in individuals with hair thinning, offering a safe and effective treatment option compared to placebo.
Read the whole study here.
Is Extracorporeal Shockwave Therapy Effective Against Erectile Dysfunction?
Numerous studies have been conducted to evaluate the efficacy of ESWT in treating erectile dysfunction, and the results have been promising.
Observed Clinical Improvements in ED Symptoms
Gruenwald, et al. (2013) performed a double-blind randomized control trial that supported the potential of using ESWT as a standard treatment for ED. Their study demonstrated positive changes in erectile function and penile blood flow after LI-ESWT. It also showed that ESWT is safe, well-tolerated, and has no adverse side effects.
This observation is also consistent with the umbrella review of five systematic reviews and meta-analyses conducted by Medrano-Sánchez, et al. (2024), in which the studies showed significant clinical improvements in ED symptoms based on the parameters set in the International Index of Erectile Function-Erectile Function (IIEF-EF) and the Erection Hardness Score (EHS) scale.
Efficacy of ESWT Against Tadalafil (A PDE5 Inhibitor)
A study by Zanaty et al. (2022), demonstrated that the clinical improvements of patients with ED who underwent ESWT were comparable to those who took 20 mg of tadalafil, a PDE-5 inhibitor. Safety outcomes were also demonstrated to be better for those who underwent ESWT.
However, the study noted that these were still short-term observations and that more studies must be done to determine the long-term effects of ESWT on ED.
Safety Profile of ESWT for ED
Aside from promising results regarding its effectiveness, the safety profile of ESWT is also good, with only mild side effects reported by those who have undergone the therapy. Side effects of both focused SWT and radial SWT are minimal and subside a few days after the treatment. These reported side effects include bruising, swelling, and numbing or pain in the treated area.
You Are Not Just Human—You’re a Living Galaxy
Did you know that inside your body live trillions of microorganisms—and they carry millions of genes that don’t even belong to you?
Welcome to your gut microbiome—a vast, intelligent ecosystem made up of bacteria, fungi, viruses, and other microbes that outnumber your own human cells. Their genes, collectively called the microbiome genome, make up a second genetic code—one that profoundly influences your hormones, metabolism, immune function, and even your longevity.
In fact, scientists estimate that the genes in your microbiome outnumber your human genes by 100 to 1.
These microbial genes:
• Train your immune system
• Produce key neurotransmitters like
serotonin and GABA
• Regulate your estrogen, testosterone, and
cortisol
• Influence how you store fat, burn energy,
and age
And here’s the mind-blowing part: your microbiome is as unique as your fingerprint. What you eat, how you sleep, the medications you’ve taken, even how you were born (C-section vs. vaginal)—all shape your internal ecosystem.
This isn’t just about digestion—it’s about total body optimization. Supporting your microbiome can mean more energy, better hormone balance, improved mood, and a longer, healthier life.
When you feed your microbes well, they become your most powerful wellness allies.
Bremelanotide for Treatment of Female Hypoactive Sexual Desire
Edinoff AN, Sanders NM, Lewis KB, Apgar TL, Cornett EM, Kaye AM, Kaye AD. Neurol Int. 2022 Jan 4;14(1):75-88. doi: 10.3390/neurolint14010006. PMID: 35076581; PMCID: PMC8788464.
Study Focus: Investigated Bremelanotide's effectiveness in treating Female Hypoactive Sexual Desire Disorder (HSDD).
Population: Premenopausal women diagnosed with HSDD.
Method: Randomized, double-blind, placebo-controlled trial.
Dosage: Participants self-administered Bremelanotide before anticipated sexual activity.
Key Findings:
Significant improvement in sexual desire compared to placebo.
Increase in number of satisfying sexual events reported.
Positive impact on overall sexual function and arousal.
Side Effects: Mild to moderate nausea most common; generally well tolerated.
Conclusion: Bremelanotide is an effective and safe treatment option for women with HSDD, offering improved sexual desire and satisfaction.
Intranasal administration with NAD+ profoundly decreases brain injury in a rat model of transient focal ischemia
Weihai Ying 1, Guangwei Wei, Dongmin Wang, Qing Wang, Xiannan Tang, Jian Shi, Peng Zhang, Huafei Lu
Affiliations Expand
PMID: 17127275
DOI: 10.2741/2267
NAD+ treatment significantly reduced the extent of brain injury.
The study was conducted in a rat model of transient focal ischemia.
NAD+ showed neuroprotective effects during ischemic events.
The intranasal route provided a non-invasive and effective means to deliver NAD+.
Results suggest potential for NAD+ in treating ischemic brain injuries.
Findings support further research into NAD+ for stroke and related conditions.
Weihai Ying 1, Guangwei Wei, Dongmin Wang, Qing Wang, Xiannan Tang, Jian Shi, Peng Zhang, Huafei Lu
Read the study here: https://pubmed.ncbi.nlm.nih.gov/17127275/
What are Stem Cells?
Nature: Living, undifferentiated cells capable of self-renewal and transforming into specialized cell types (such as muscle, blood, or brain cells).
Function: Act as a cellular “repair system.” They divide and remain as stem cells or become specialized cells, allowing tissue repair and regeneration. Stem cells can intrinsically differentiate into specialized cell types—such as muscle, nerve, or blood cells—allowing them to “replace” or replenish damaged tissue cells directly.
Therapeutic Use: In stem cell therapy, new stem cells are introduced into the body to replace or repair damaged tissues. The treatment leverages the cells’ ability to multiply and differentiate, fostering healing long after administration.
Size: Much larger than exosomes (average 13,000 nanometers).
Replication: Stem cells can proliferate and, in rare cases, pose a risk of uncontrolled growth (tumor formation). Because they can become various cell types, stem cell therapies may offer robust tissue regeneration, particularly where large-scale tissue restoration is needed.
What are Exosomes?
Nature: Tiny, non-living extracellular vesicles (about 30–150 nanometers) released by cells, including stem cells. Exosomes are “vesicles” or “carrier pods” containing proteins, growth factors, RNA, and signaling molecules.
Function: Serve as intercellular messengers, transferring molecular cargo (like proteins and genetic material) from one cell to another to influence the behavior of recipient cells. Exosomes do not become new tissue but instead carry signaling molecules (including proteins and RNA) that influence the behavior of recipient cells. They enhance existing cells’ repair processes, modulate inflammation, and promote tissue healing by sending “instructions” to resident cells.
Therapeutic Use: Exosome therapy uses isolated exosomes (often from stem cells) to promote tissue repair by enhancing cell-to-cell communication and facilitating healing signals, without introducing whole cells or the risks associated with them (e.g., immune rejection, uncontrolled cell division).
Size: Exosomes are about 100 times smaller than stem cells.
Replication: Do not replicate, and cannot become malignant, as they are not cells.
No Direct Replacement: Exosomes alone do not create new tissue; they orchestrate and enhance the body’s healing mechanisms by acting as cellular messengers.
Reduced Risk, Faster Effects: While exosomes can stimulate quicker recovery due to their rapid signaling capabilities, their effects are generally more transient and supportive, not replacing actual lost or damaged cells.
Stable Gastric Pentadecapeptide BPC 157 and Wound Healing
Seiwerth S, Milavic M, Vukojevic J, Gojkovic S, Krezic I, Vuletic LB, Pavlov KH, Petrovic A, Sikiric S, Vranes H, Prtoric A, Zizek H, Durasin T, Dobric I, Staresinic M, Strbe S, Knezevic M, Sola M, Kokot A, Sever M, Lovric E, Skrtic A, Blagaic AB, Sikiric P. Stable Gastric Pentadecapeptide BPC 157 and Wound Healing. Front Pharmacol. 2021 Jun 29;12:627533. doi: 10.3389/fphar.2021.627533. PMID: 34267654; PMCID: PMC8275860.
The study on gastric pentadecapeptide BPC 157 demonstrated significant and promising positive effects on wound healing processes. BPC 157 was found to accelerate tissue repair by promoting angiogenesis, enhancing cell migration, and effectively modulating inflammatory responses. This peptide notably improved the regeneration of various types of tissues, including skin, muscle, and tendon, while simultaneously reducing fibrosis and minimizing scar formation. These compelling results strongly suggest that BPC 157 holds potent therapeutic potential for significantly enhancing wound healing and recovery outcomes in various clinical settings.
Read the full article here.
Prolonged stimulation of growth hormone (GH) and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GH-releasing hormone, in healthy adults
Single injection of CJC-1295 caused dose-dependent increases in mean plasma growth hormone (GH) levels, with elevations ranging from 2- to 10-fold lasting 6 days or more.
Mean plasma insulin-like growth factor 1 (IGF-I) levels rose by 1.5- to 3-fold, sustained for 9 to 11 days after a single dose.
The half-life of CJC-1295 was estimated between 5.8 and 8.1 days.
Multiple doses of CJC-1295 maintained mean IGF-I levels above baseline for up to 28 days.
No serious adverse reactions were reported, suggesting a favorable safety profile for CJC-1295.
Read the full article here.
Are Hormones Safe?
A comprehensive Yale University study tracking 7 million women over 12 years found that those who used estrogen therapy for at least five years experienced a 33% lower risk of developing breast, ovarian, lung, and colorectal cancers.
Key Findings:
22% Reduction in Breast Cancer Incidence: Use of conjugated equine estrogen (CEE) alone was linked to a 22% decrease in new breast cancer cases.
40% Reduction in Breast Cancer Mortality: CEE alone correlated with a 40% reduction in deaths from breast cancer.
28% Increased Risk with Combination Therapy: Women using a combination of CEE and medroxyprogesterone acetate (MPA) showed a 28% higher risk of breast cancer.
33% Lower Risk of Multiple Cancers: Long-term estrogen therapy significantly reduced the risk of breast, lung, and colorectal cancers by one-third.
These findings highlight the potential protective effect of estrogen-only therapy on certain cancer risks, emphasizing the importance of personalized hormone treatment decisions.
__________________________________________________________________________
Benefits of Lower-Dose and Non-Oral Hormone Replacement Therapy (Manson et al., 2024):
Estrogen therapy alone is linked to a 19% reduction in overall mortality. Additionally, it lowers the risks of breast cancer, colorectal cancer, congestive heart failure, venous thrombosis, embolisms, atrial fibrillation (AFib), acute myocardial infarctions, and dementia.
ACOG Stance on Menopausal Hormone Therapy and Breast Cancer (Levy & Simon,
2024):
Conjugated Estrogen-Alone Trial: In this study, CEE was linked to a 45% statistically significant reduction in breast cancer mortality after 18 years of careful follow-up and observation.Nationwide Finnish Comparative Study: Estradiol (E2) alone, when used for more than 10 years, is safe for the breast. Even when combined with a progestin, there was a 50% breast cancer mortality risk reduction compared to placebo. There is no conclusive evidence showing that HT causes breast cancer, and if anything, HT has been shown to have a null or protective effect.\
Exposure to Exogenous Estrogen (ERT) Prevents Breast Cancer (Manyonda et al., 2022):
The WHI study of estrogen replacement therapy (ERT) using conjugated equine estrogens (CEE) in women with a prior hysterectomy, compared to placebo, supports a clear conclusion: exposure to exogenous estrogen (ERT) significantly reduces the risk of developing breast cancer in this patient group.
Use of Menopausal Hormone Therapy Beyond Age 65 Years and Its Effects on Women's
Health Outcomes by Types, Routes, and Doses (Baik et al., 2024):
Using estrogen therapy alone after age 65 significantly lowers the risk of breast cancer (16%), lung cancer (13%), and colorectal cancer (12%) compared to not using or stopping hormone therapy after 65.
Finnish Study 2016: Lower breast cancer death risk in women using postmenopausal hormone therapy: A Finnish study of 489,105 women from 1994 to 2009 found that hormone therapy users had a reduced risk of dying from breast cancer. The risk was lower for those using hormones up to 5 years (risk 0.56), 5 to 10 years (0.46), or over 10 years (0.62).
2017 Study: Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal
women: a systematic review and meta-analysis.
1. 2. 3. 4. Estradiol-only therapy does not increase breast cancer risk. The risk depends on the type of progestogen used. Combining estradiol with medroxyprogesterone, norethisterone, or levonorgestrel (these are NOT bio identical hormones) raises breast cancer risk. Combining estradiol with natural progesterone or dydrogesterone does not increase risk.
WHI (2) Re-analysis 2019 By Original Authors:
1. 2. Women on estrogen only had 23% decreased incidence of breast cancer.
Women on estrogen only had 44% decreased fatality.
References:
● Chlebowski, Rowan T ., et al. (2020).
"Association of menopausal hormone therapy with
breast cancer incidence and mortality during long-term follow-up of the women’s health
initiative randomized clinical trials.
" JAMA, 324(4): 369-380.
● Manson, JoAnn E., et al. (2024).
"Benefits and risks of hormone therapy for
postmenopausal women: A focus on the new WHI findings.
" Menopause, 31(3):
259-269.
● Levy, Deborah, and Joseph Simon. (2024).
"Menopausal hormone therapy and
breast cancer risk: A reappraisal of the evidence.
" Obstetrics and Gynecology
Clinics of North America, 48(4): 657-670.
● Manyonda, Isaac, et al. (2022).
"Estrogen replacement therapy for the prevention of
breast cancer: current status and future directions.
" Endocrine-Related Cancer, 29(9):
R203-R215.
● Baik, Sarah H., et al. (2024).
"Use of menopausal hormone therapy beyond age
65 years and its effects on women’s health outcomes by types, routes, and
doses.
" Maturitas, 146: 1-9.
Cancer Risk - Progesterone
Recent studies show that the type of progesterone in hormone therapy affects cancer risk, especially breast cancer. Synthetic progestins like medroxyprogesterone acetate (MPA) may increase breast cancer risk when used with estrogen. Bioidentical progesterone, however, tends to have no effect or may protect against it.
Key Findings:
Synthetic Progestins: Synthetic progestins, such as MPA, have been associated with an increased risk of breast cancer when combined with estrogen in hormone therapy.
Bioidentical Progesterone: Bioidentical progesterone does not elevate breast cancer risk and may offer protective effects during hormone therapy.
Comparative Studies: Research comparing the two indicates that women receiving synthetic progestins face a higher likelihood of breast cancer than those using bioidentical progesterone.
References:
● Fournier, Agnès, et al. (2008).
"Breast cancer risk associated with different types of
hormone replacement therapy in the E3N-EPIC cohort.
" Journal of Clinical Oncology,
26(8): 1260-1268.
● Stute, Petra, et al. (2016).
"Progesterone use in postmenopausal women and breast
cancer risk.
" Climacteric, 19(4): 316-324.
● Campagnoli, Carlo, et al. (2005).
"Progestins and progesterone in hormone replacement
therapy and the risk of breast cancer.
" Journal of Steroid Biochemistry and Molecular
Biology, 96(2): 95-108.
Cancer Risk - Testosterone
Testosterone therapy has been closely scrutinized for its potential impact on cancer risk, particularly concerning prostate cancer in men and breast cancer in women. However, recent clinical studies and research indicate that testosterone therapy, when properly managed and monitored by healthcare professionals, does not significantly increase the risk of developing cancer. In fact, some evidence suggests that it may even contribute to a reduced incidence of certain types of cancers, highlighting the importance of personalized treatment plans and ongoing medical supervision.
References:
● Morgentaler, Abraham. (2013).
"T estosterone therapy in men with prostate
cancer: scientific and ethical considerations.
" The Journal of Urology, 189(1):
S26-S33.
● Glaser, Rebecca, and Constantine Dimitrakakis. (2013).
"T estosterone therapy in
women: myths and misconceptions.
" Maturitas, 74(3): 230-234.
● Marks, Leonard S., et al. (2006).
"Effect of testosterone replacement therapy on
prostate tissue in men with late-onset hypogonadism: a randomized controlled trial."
JAMA, 296(19): 2351-2361.
The Effect of Platelet-Rich Plasma in Hair Regrowth
The study titled "The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial" by Pietro Gentile et al. investigated the impact of platelet-rich plasma (PRP) therapy on hair regrowth. The researchers conducted a controlled trial comparing PRP treatment to a placebo in patients experiencing hair loss. They found that PRP significantly improved hair density and thickness compared to the placebo group. The study concludes, "PRP represents a safe and effective treatment for hair regrowth, showing significant improvement in hair count and hair thickness." This suggests that PRP could be a promising option for individuals seeking non-surgical hair restoration treatments.
Read the full study here.
Low-Dose Naltrexone (LDN) Research Summary
Low-Dose Naltrexone (LDN) has been the subject of multiple studies exploring its potential in modulating the immune system, reducing inflammation, and improving outcomes across various chronic conditions. Below is a summary of published research findings.
Cancer Risk
Clinical trials published in Cureus
Proposed mechanisms suggest that LDN may affect tumor cells, including non-small cell lung cancer (NSCLC), by enhancing immune system activity at the cellular level.Mouse study in ScienceDirect
Demonstrated that low-dose naltrexone inhibits colorectal cancer progression and promotes apoptosis (programmed cell death).Review in International Immunopharmacology
Found that LDN increases production of methionine enkephalin (MENK), an opioid growth factor that regulates cell proliferation and may help inhibit tumor growth.
Inflammation
10-week crossover trial in Biomedicine
Showed LDN reduced levels of pro-inflammatory cytokines, including TNF-α and TGF-β, which are involved in systemic inflammation.Review in Clinical Rheumatology
Highlighted multiple studies showing that LDN reduces oxidative stress and markers of inflammation such as ESR (erythrocyte sedimentation rate).
Fibromyalgia
Two pilot studies
Found that LDN is a safe, well-tolerated, and effective treatment option for fibromyalgia symptom reduction.Cohort study
Reported more than 30% symptom improvement compared to placebo.Randomized, double-blind crossover trial
Observed a significant reduction in baseline pain among patients taking LDN compared to placebo.
Crohn’s and Ulcerative Colitis
Study in the American Journal of Gastroenterology
Found that 81% of Crohn’s disease patients taking 4.5mg LDN for 4 weeks responded to therapy, with 67% achieving remission. The main side effect was mild sleep disturbance.
Multiple Sclerosis
Study in the Journal of Clinical Psychopharmacology
Involving 215 MS patients, showed 77% experienced no side effects and 60% reported improved quality of life when taking LDN.
Fertility
Study of women with ovarian failure
Reported a 74% pregnancy and live birth rate among women treated with LDN.
Weight Loss
Study in hyperandrogenic women
Showed LDN lowered fasting insulin levels by up to 40%.Study in obese women
Found that LDN may help increase growth hormone levels, supporting fat loss and lean body mass development.LDN + Bupropion combination study
Demonstrated greater weight reduction compared to Bupropion alone.
Safety and Efficacy of Naltrexone for Weight Loss in Adult Patients – A Systematic Review
Introduction
Obesity remains a significant public health challenge worldwide, contributing to increased morbidity and mortality related to cardiovascular disease, diabetes, and other comorbidities. Pharmacological interventions, such as naltrexone, have been explored for their potential role in weight management. This systematic review evaluates the safety and efficacy of naltrexone in adult patients seeking weight loss.
Methods
A comprehensive literature search was conducted across major medical databases, including PubMed, Embase, and Cochrane Library, focusing on randomized controlled trials (RCTs), observational studies, and meta-analyses published up to 2025. Keywords included "naltrexone," "weight loss," "obesity," and "adults." Studies were selected based on relevance, quality, and sample size, with emphasis on safety profiles and efficacy outcomes.
Results
Efficacy
Weight Reduction: Across multiple RCTs, naltrexone, especially when combined with bupropion, demonstrated statistically significant weight loss compared to placebo. Average weight loss ranged from 4-6% of baseline body weight over 24-52 weeks.
Appetite Suppression: Naltrexone’s mechanism targeting the hypothalamic pathways and opioid receptors contributes to decreased appetite and reduced food cravings, supporting sustained weight loss.
Metabolic Improvements: Some studies reported improvements in metabolic parameters, including glucose regulation and lipid profiles, though results varied.
Safety
Adverse Effects: Common adverse events associated with naltrexone include nausea, headache, dizziness, and gastrointestinal disturbances. These effects were generally transient and mild to moderate in severity.
Serious Events: Serious adverse events were rare. However, naltrexone is contraindicated in patients with acute hepatitis or liver failure due to potential hepatotoxicity.
Tolerance and Discontinuation: A proportion of patients discontinued treatment due to side effects, underscoring the need for monitoring and individualized dose adjustments.
Discussion
Naltrexone, particularly in combination with bupropion, offers a viable pharmacological option for weight management in adults with obesity or overweight conditions. The drug’s dual action on the central nervous system mediates appetite suppression and reward mechanisms related to food consumption. While generally safe, clinicians must assess liver function prior to therapy and monitor patients regularly to mitigate adverse effects.
Conclusion
Naltrexone is effective and safe for promoting weight loss in adult patients when used appropriately and under medical supervision. It should be integrated into comprehensive weight management plans that include lifestyle modification. Further long-term studies are warranted to fully elucidate its benefits and risks.
Recommendations
Prior evaluation of liver function is essential before initiating naltrexone.
Combination therapy with bupropion is preferred to enhance efficacy.
Continuous monitoring for side effects should be implemented to improve treatment adherence.
Consideration of patient-specific factors and contraindications is crucial for safe prescribing.
Vitality Cove remains committed to evidence-based approaches in hormone health and wellness, supporting optimal patient outcomes through the prudent use of pharmacotherapy in weight management.
Citation: Kulak-Bejda A, Bejda G, Waszkiewicz N. Arch Med Sci. 2020;17(4):940–953. PMCID: PMC8314402.
Goal: Systematically review the evidence on safety and efficacy of naltrexone alone or naltrexone + bupropion for weight loss in adults.
Methods: searched Medline/PubMed/Embase/Cochrane (1966→Jan 2018). Included meta-analyses, randomized controlled trials (RCTs), controlled/uncontrolled trials, cohorts, open-label studies.
What they included: 191 records screened → 14 studies met inclusion (1 prior meta-analysis, 10 RCTs, 3 non-randomized studies). Most trials evaluated the naltrexone + bupropion combination (the commercial NB product), not naltrexone monotherapy.
Key efficacy findings (pooled / trial-level):
A prior meta-analysis within the review found naltrexone/bupropion vs placebo produced about 5.0 kg greater weight loss (placebo-subtracted) and markedly increased odds of achieving ≥5% weight loss (OR ≈ 3.96).
Individual trials reported clinically meaningful percent weight change (one trial with comprehensive lifestyle intervention reported ~8.5% weight reduction at 26 weeks with NB + lifestyle vs control).
Safety / tolerability:
Overall well tolerated, most common adverse event was nausea.
The combination had a higher rate of treatment discontinuation for adverse events than placebo in some analyses.
Large cardiovascular outcomes trial included in the review did not show increased MACE with NB; hazard ratio for MACE was not statistically increased (HR ≈ 0.88 with wide CI).
Other notes from the review:
Some psychiatric and eating-behavior signals: NB showed benefit in binge-eating symptoms in one study and helped attenuate antipsychotic-associated weight gain in at least one trial.
The authors emphasize individualized treatment because adverse-event sensitivity varies and can lead to dropouts. Overall conclusion: naltrexone/bupropion is an effective option for weight loss when added to lifestyle measures and appears reasonably safe in trial populations.